These are the images, diagnosis and treatment of the disease caused by Human Papillomavirus Infection and Genital Warts. This is a Viral Sexually Transmitted Diseases.
Anogenital infection with human papillomavirus (HPV), long considered an inconvenient but benign condition, has emerged as one of the most important as well as one of the most common STDs. HPV is ubiquitous, and at least 80% of sexually active persons acquire at least one anogenital infection during their sexually active lives. Reflecting both high prevalence and efficient sexual transmission, up to half of all persons are infected within their first three lifetime sex partners, and the prevalence of genital HPV ranges from 20 to 50% in most sexually active populations. Over 100 HPV types are known, of which about 40 usually infect the genital tract or anus and are transmitted primarily if not exclusively by sexual contact. Anogenital HPV strains comprise two broad classes, based on their association with cancer and premalignant neoplasia. “Low-risk” HPV types are infrequently implicated in cancer or highgrade dysplasia, although they often cause low-grade dysplasia that regresses without treatment. Among the low-risk types are HPV-6 and 11, which cause 85 to 90% of anogenital warts. “High-risk” HPV types, such as HPV-16, 18, 31, 45, and several others, cause dysplasia and cancer of the cervix, anus, penis, and vulva. HPV-16 and 18 account for 65 to 70% of cervical cancers and precancerous dysplasia, with remarkably similar prevalences in all populations worldwide; there is somewhat greater variation in the frequencies of other oncogenic types like HPV-31, 45, and others. Among asymptomatic persons tested for genital HPV infection, HPV-16 is the most common type overall in both the general population and those at high risk for STD. The type distributions are similar in both sexes and for vaginal, penile, and anal HPV infections.
Anogenital HPV-6 and 11 infections result in overt warts in about two-thirds of infected persons, but most other anogenital HPV infections remain subclinical, causing neither symptoms n dysplasia. Although HPV DNA may persist for life in squamous epithelium, detectable infection using currently available technologies usually resolves spontaneously within 6–12 months for low-risk HPV types and 12–24 months for HPV-16 and 18. However, late recurrence is not rare and accounts for some genital warts and probably most cases of dysplasia or cancer in persons ≥30 years old. Reinfection with the same HPV type appears to be infrequent, probably owing to acquired immunity. Because no treatment has been shown to eliminate the virus from infected skin and mucous membranes, the primary goals for clinical management are elimination of symptomatic warts, treatment of malignancy and premalignant changes, and counseling to limit psychosocial distress.
For half a century, prevention of HPV morbidity in industrialized countries has been based primarily
on cervical cytology to detect and treat dysplasia and carcinoma in situ. Since 1960, annual rates of cervical cancer in the United States and most industrialized countries have declined from 30 to 40 cases to 8 to 10 cases per 100,000 women. Cytology-based prevention is beyond the means of most developing countries, but detection of high-risk HPV by nucleic acid amplification tests (NAATs) (e.g., using self-collected vaginal swabs) now has promise for cervical cancer prevention. Most important, the means are now at hand for primary prevention of anogenital HPV infection through immunization. The development of effective vaccines against selected HPV types—biologically among the most effective vaccines ever produced—was a milestone in both STD prevention and cancer prevention. Bivalent (HPV-16 and 18) and quadrivalent (HPV-6, 11, 16, and 18) vaccines are currently available, and vaccines with expanded strain coverage are in development. Routine immunization is now recommended for all young women and girls. Because risk for HPV begins immediately after onset of sexual activity and incident infections are common in sexually active teens, maximum benefit from immunization requires vaccination of girls prior to sexual debut, ideally before age 12. Immunization of boys and men also is increasingly advised, both to limit HPV morbidity in men, especially anal cancer, and to help prevent transmission of HPV to women. Condoms significantly reduce the risk of anogenital HPV infection but protection is incomplete, and all prevention strategies other than immunization have limited efficacy, partly because multiple anogenital HPV strains remain ubiquitous and efficiently transmitted.
Oral infections with genital HPV types are not uncommon, but most are subclinical. The rates of tonsilar and other posterior pharyngeal cancers associated with HPV-16 are rising in the United States and perhaps elsewhere, perhaps the result of rising frequencies of orogenital sex in recent decades. However, these malignancies remain rare, and many cases continue to occur in persons >50 years old with other risk factors like tobacco and alcohol abuse. Even if HPV-16 is the direct cause of these malignancies, the link to sexual acquisition of oral HPV infection is tenuous, and it remains to be seen whether immunization will protect against HPV-16-related pharyngeal cancer. Research is evolving rapidly and clinicians should be alert to new developments and improved understanding of oral HPV infection and its consequences.
This chapter primarily addresses the epidemiologic and clinical aspects of anogenital warts. Other sources should be consulted for diagnosis and management of HPV-related malignancies and premalignant lesions, including management of women with abnormal cervical cytology
EPIDEMIOLOGY
Incidence and Prevalence
• Estimated incidence 6 million anogenital HPV infections annually in the United States
• In most countries, >80% of sexually active persons acquire one or more genital HPV infections
• Prevalence 20–50% in most sexually active populations age 15–40 years in the United States
• In the United States, 6% of all persons (males 4%, females 7%) report past history of genital warts, including 11% of persons with >10 lifetime sex partners; genital warts account for 300,000–400,000 physician visits per year
• Population-based modeling studies in Scandinavia suggest lifetime risk of genital warts approximates 15–20%
• Overall rates are similar in most populations worldwide
• Anogenital HPV is almost equally prevalent in populations at low and high risk for other STDs
Transmission
• Sexual contact, probably enhanced by friction or microtrauma
° Most cases transmitted by vaginal or anal intercourse
° Genital to oral transmission may be frequent, but symptomatic oral HPV is uncommon
° Oral to genital transmission probably is uncommon
° Transmission by hand-genital contact, directly or by exchange of genital secretions, probably is uncommon, but may explain some cases in infected persons who deny other sexual exposures
• Autoinoculation to nongenital sites may occur, but clinical manifestations are rare at sites other than genitals or anus
• Perinatal transmission to newborns during vaginal delivery can cause respiratory papillomatosis; infrequent but potentially serious
• Fomite transmission occurs rarely if ever
Age
• Most infections acquired by persons from age of sexual debut to 30 years old
Sex
• No specific predilection known for HPV infection per se
• More frequently diagnosed in women than men due to Pap screening and more frequent attendance
for health care
Sexual Orientation
• No predilection for HPV infection per se
• Anogenital infection with both high- and low-risk HPV is common in both MSM and WSW
• Among MSM, annual anal cancer rate approximates 35–40 per 100,000 (equivalent to cervical cancer rate in absence of routine Pap smears)
Other Risk Factors
• Circumcision in men is partly protective; warts and subclinical penile HPV infection are less frequent in circumcised than uncircumcised men
• Cellular immunodeficiency (e.g., advanced HIV infection) is associated with recrudescence of warts, atypical locations (e.g., oral and facial), and probably accelerated progression of dysplasia and cancer.
HISTORY
Incubation Period
• Exophytic warts typically appear 2–12 months after exposure (mean 6–8 months)
• Cervical dysplasia can develop within several weeks of acquiring HPV; severe dysplasia and, rarely,
carcinoma in situ can develop without intervening mild dysplasia
• Progression to invasive cancer typically requires 5–30 years, but can occur within 1 year
Symptoms
• High-risk HPV infections usually are asymptomatic
• Anogenital warts are the most common symptom; 60–70% of HPV-6 or 11 infections result in overt warts
• HPV infection per se rarely if ever causes pruritus, burning, or similar symptoms (notwithstanding common perceptions by some patients)
• Large or traumatized warts may ulcerate or become secondarily infected, with itching, pain, discharge, or malodor
• Urethral warts in men may cause altered urine stream and, rarely, outflow obstruction
• External genital or anal neoplastic lesions, such as vulvar intraepithelial neoplasia (VIN), and comparable lesions of the vagina (VaIN), penis (PIN), or anus (AIN)
• Respiratory papillomatosis in infants, and rarely in adults, can cause hoarseness and, in advanced cases, airway obstruction
Epidemiologic History
• Most patients with new anogenital warts acknowledge new sexual partnerships in preceding 1–2 years
• Many persons with subclinical HPV infection lack recent behavioral STD risks
• Estimated incidence 6 million anogenital HPV infections annually in the United States
• In most countries, >80% of sexually active persons acquire one or more genital HPV infections
• Prevalence 20–50% in most sexually active populations age 15–40 years in the United States
• In the United States, 6% of all persons (males 4%, females 7%) report past history of genital warts, including 11% of persons with >10 lifetime sex partners; genital warts account for 300,000–400,000 physician visits per year
• Population-based modeling studies in Scandinavia suggest lifetime risk of genital warts approximates 15–20%
• Overall rates are similar in most populations worldwide
• Anogenital HPV is almost equally prevalent in populations at low and high risk for other STDs
Transmission
• Sexual contact, probably enhanced by friction or microtrauma
° Most cases transmitted by vaginal or anal intercourse
° Genital to oral transmission may be frequent, but symptomatic oral HPV is uncommon
° Oral to genital transmission probably is uncommon
° Transmission by hand-genital contact, directly or by exchange of genital secretions, probably is uncommon, but may explain some cases in infected persons who deny other sexual exposures
• Autoinoculation to nongenital sites may occur, but clinical manifestations are rare at sites other than genitals or anus
• Perinatal transmission to newborns during vaginal delivery can cause respiratory papillomatosis; infrequent but potentially serious
• Fomite transmission occurs rarely if ever
Age
• Most infections acquired by persons from age of sexual debut to 30 years old
Sex
• No specific predilection known for HPV infection per se
• More frequently diagnosed in women than men due to Pap screening and more frequent attendance
for health care
Sexual Orientation
• No predilection for HPV infection per se
• Anogenital infection with both high- and low-risk HPV is common in both MSM and WSW
• Among MSM, annual anal cancer rate approximates 35–40 per 100,000 (equivalent to cervical cancer rate in absence of routine Pap smears)
Other Risk Factors
• Circumcision in men is partly protective; warts and subclinical penile HPV infection are less frequent in circumcised than uncircumcised men
• Cellular immunodeficiency (e.g., advanced HIV infection) is associated with recrudescence of warts, atypical locations (e.g., oral and facial), and probably accelerated progression of dysplasia and cancer.
HISTORY
Incubation Period
• Exophytic warts typically appear 2–12 months after exposure (mean 6–8 months)
• Cervical dysplasia can develop within several weeks of acquiring HPV; severe dysplasia and, rarely,
carcinoma in situ can develop without intervening mild dysplasia
• Progression to invasive cancer typically requires 5–30 years, but can occur within 1 year
Symptoms
• High-risk HPV infections usually are asymptomatic
• Anogenital warts are the most common symptom; 60–70% of HPV-6 or 11 infections result in overt warts
• HPV infection per se rarely if ever causes pruritus, burning, or similar symptoms (notwithstanding common perceptions by some patients)
• Large or traumatized warts may ulcerate or become secondarily infected, with itching, pain, discharge, or malodor
• Urethral warts in men may cause altered urine stream and, rarely, outflow obstruction
• External genital or anal neoplastic lesions, such as vulvar intraepithelial neoplasia (VIN), and comparable lesions of the vagina (VaIN), penis (PIN), or anus (AIN)
• Respiratory papillomatosis in infants, and rarely in adults, can cause hoarseness and, in advanced cases, airway obstruction
Epidemiologic History
• Most patients with new anogenital warts acknowledge new sexual partnerships in preceding 1–2 years
• Many persons with subclinical HPV infection lack recent behavioral STD risks
PHYSICAL EXAMINATION
• Genital wart morphology
° Condylomata acuminata (singular, condyloma acuminatum): Moist or partially keratinized surfaces (e.g., introitus, anus, under foreskin); typical “cauliflower” appearance; central venules in fronds
may be observed with handheld magnification or colposcopy
° Keratotic warts: Horny, often cauliflower-like appearance, typically on dry skin, e.g., penile shaft, scrotum, labia majora
° Papular warts: Smooth surface, less horny than keratotic warts
° Flat warts: Macular or faintly raised; usually invisible to naked eye
• Anatomic sites are primarily those most subject to friction during sex
° Penile glans or shaft, especially under foreskin of uncircumcised men
° Vaginal introitus, labia minora
° Anus: Anal warts most commonly observed in MSM, acquired by receptive anal sex; also frequent in women (with or without anal sex); less frequently but nevertheless common in heterosexual men (probably by autoinoculation or contiguous spread)
° Urethral, vaginal, cervical, or rectal mucosa
° Perigenital areas such as scrotum, labia majora, or groin are less frequently involved, but not rare
• Visual inspection in good light, sometimes aided by magnification, usually is sufficient for accurate
diagnosis of anogenital warts
• Recognition or suspicion of other HPV-related anogenital lesions, such as overt anogenital cancer,
Bowenoid papulosis, VIN, PIN, AIN, or VaIN, requires considerable expertise and confirmation by
biopsy
• Application of 3% acetic acid may highlight HPV-infected skin or mucosa (“acetowhitening”) to identify subclinical infection, but both false-positive and false-negative results are common; not recommended except by well-trained experts
• Colposcopy is routine in evaluation of women with cervical dysplasia or other Pap smear abnormalities and can identify cervical or vaginal warts
• Anoscopy may reveal rectal warts, but importance of detecting or treating asymptomatic rectal warts is unknown
• Screen patients with newly diagnosed anogenital warts or abnormal cytology for other common STDs and HIV
LABORATORY DIAGNOSIS
Cytology, especially of the cervix and increasingly on anal specimens, is time-honored and remains the primary method to identify presumed HPV infections of mucosal surfaces, especially the cervix. Nucleic acid amplification tests (NAATs) are available to detect HPV, typically classified as high or low risk (usually without determination of individual HPV types), to guide management of patients with abnormal Pap smears, and may have an independent role in screening to identify persons at risk for cervical or anal cancer where Pap smears are not readily available, especially in developing countries. Currently available HPV NAATs are neither approved nor recommended to diagnose asymptomatic HPV infection in males or for anatomic sites other than the cervix, vagina, and anus.
• Typical changes on cervical cytology (Pap smear):
° High-grade and low-grade squamous intraepithelial lesions (HSIL and LSIL, respectively) and carcinoma in situ always indicate HPV infection
° HPV is present in about half of patients with atypical squamous or glandular cells of undetermined significance (ASCUS, AGUS)
• HPV NAAT can help guide management of patients with selected cytologic abnormalities, e.g., observation and repeat Pap smear versus early colposcopy and biopsy; may supplant Pap smear in some settings (research in progress)
• Screening MSM for anal dysplasia and cancer with anal cytology and/or HPV NAAT is advocated by some investigators but not currently recommended for routine use
• Type-specific HPV antibody tests are used for research but currently have insufficient performance
for reliable clinical use
DIFFERENTIAL DIAGNOSIS OF ANOGENITAL WARTS
Visual diagnosis is adequate for most exophytic warts, but biopsy is indicated for pigmented and other atypical lesions, those that do not respond to treatment or have clinical appearances suggestive of malignancy or premalignant changes, such as Bowenoid papulosis, leukoplakia, and penile, vulvar, vaginal, and anal intraepithelial neoplasia (PIN, VIN, VaIN, and AIN, respectively) and overt squamous cell cancers. Pathologic and normal findings commonly mistaken for anogenital warts include molluscum contagiosum (see Chap 10), condylomata lata of syphilis, pearly penile papules, skin tags, Tyson glands, and other anatomic variants.
TREATMENT OF ANOGENITAL WARTS
Principles
The primary goal of treatment is to eradicate overt warts; no available therapy has been shown to eradicate HPV. Eliminating visible warts might reduce the risk of sexual transmission, but no data are available. Surgical excision, laser cautery, or electrocautery immediately ablate visible warts. Other modalities (cryotherapy, podophyllin, podofilox, sinecatechins, tri- or bichloroacetic acid, imiquimod) have 60–80% efficacy within several weeks, but the therapeutic response is highly variable from one patient to another.
Recurrence of warts within 2–3 months is equally common with all therapies. Combination methods, such as cryotherapy plus podophyllin, speeds resolution in some patients. When therapy is unsuccessful or in event of prompt recurrence, an alternate method should be used. Other factors that influence choice of treatment include size and number of warts, anatomic site, provider experience, cutaneous versus mucosal surfaces, cost, and preference for patient-applied or provider-applied treatment. Treatment is not recommended for subclinical anogenital or oral infection that may be diagnosed by colposcopy, application of acetic acid to normal-appearing skin or mucosa, or positive NAAT for HPV
Patient-Applied Treatment of External Warts
Podofilox (Condylox) is a purified antimitotic agent derived from podophyllin, chemically related to the vinca alkaloids. Sinechatechins (Veregen) is a green tea extract that has been effective against genital warts in uncontrolled clinical trials. Imiquimod (Aldara) is an immune enhancer that stimulates local production of interferon and other cytokines, a mechanism that might be especially effective in preventing recurrent warts. However, a reduced recurrence rate has not been documented for imiquimod, response of warts typically is slower than with other patient-applied treatments, and efficacy is reduced for lesions on dry compared with moist surfaces. Safety in pregnancy is unknown for all of the recommended patient-applied regimens.
• Podofilox 0.5% solution or gel: apply to warts bid for 3 days, followed by 4 treatment-free days; repeat weekly up to 4 cycles
• Imiquimod 5% cream: apply to warts once daily at bedtime and wash off after 6–10 hours; repeat 3 times weekly for up to 16 weeks
• Sinecatechins 15% ointment: apply to warts tid until warts resolved; maximum 16 weeks
Provider-Applied Therapy of External Warts
Selection of provider-applied treatments is primarily determined by availability in particular clinical settings and provider experience, and secondarily by cost, desire for immediate ablation of warts, size of warts, and anatomic location. Cryotherapy destroys wart tissue by thermal cellular disruption. Podophyllin resin is an antimitotic agent that is selectively toxic for neoplastic tissues, including warts. Podophyllin preparations are not well standardized and may have variable efficacy; concentrations of 10–25% are available. Trichloroacetic acid and bichloroacetic acid are caustic agents that coagulate and destroy tissue proteins.
• Cryotherapy with liquid nitrogen or cryoprobe: repeat weekly until warts resolved; 2–4 treatments usually required; main side effects are local irritation and ulceration
• Podophyllin resin, 10–25% in tincture of benzoin
° Apply to warts, minimizing contact with uninvolved tissue; allow to air dry
° Wash to remove resin 1 hour after application (optional; may reduce local irritation)
° Maximum dose 0.5 mL or 10 cm2 of treated tissue (to minimize systemic absorption)
° Repeat weekly as needed; several treatments usually required
° Primary side effects are local irritation and ulceration
° Contraindicated in pregnancy
• Trichloroacetic acid or bichloroacetic acid, 80–90% solution
° Apply to warts, with care to avoid contact with uninvolved tissue
° Repeat weekly as needed
° Several treatments usually required
° Local irritation and ulceration are primary side effects
• Surgical removal by scissor excision, tangential shave excision, electrocautery, laser cautery, etc. (special training required)
• Alternative regimens include intralesional interferon, photodynamic therapy, topical cidofovir, and isotretinoin; generally less effective, poorly studied, and expensive
Treatment of Mucosal Warts
Podophyllin, podofilox, imiquimod, and sinecatechins are not recommended for use on most mucosal surfaces, except that podophyllin resin can be used to treat vaginal warts. Options for vaginal, urethral, anal, rectal, and oral warts include cryotherapy, tri- or bichloroacetic acid, or surgical excision. Surgical removal is the main treatment for cervical warts or precancerous dysplasia.
Follow-up
• Routine follow-up not indicated after visible warts resolved
• Reevaluate if warts persist or recur
Partner Management
• Partners with symptoms (e.g., suspected warts) should be evaluated; advise patients that incubation period for visible warts may be up to 2 years.
• Routine referral and examination are not indicated for asymptomatic partners of patients with anogenital warts, abnormal cervical cytology, or other evidence of HPV infection
° Partners with ongoing exposure to infected patients can be assumed to be infected
° No recommended diagnostic tests are available
° No treatment is available for subclinical infection
• Prospective (future) partners may be offered HPV immunization
PREVENTION AND COUNSELING
• Ablation of warts and treatment of cervical or anal dysplasia may reduce viral load and perhaps transmission risk, but no data are available
• Transmission risk probably is low after warts or dysplasia are successfully treated and do not recur 3-6 months after treatment
• Most HPV infections are subclinical and remain so
• Cancer and other serious complications are uncommon
• Anogenital warts and cancers are caused by different HPV types
• HPV is readily transmissible by genital and perhaps orogenital exposure
• All sexually active persons can expect to have one or more anogenital HPV infections, usually without symptoms or serious medical consequences
• Most infections resolve spontaneously, with or without treatment
• Condoms reduce risk of infection or transmission, but protection is incomplete; substantial likelihood of HIV infection persists in consistent condom users
• Partners who have been sexually active with the index patient prior to diagnosis should assume they are infected; they should be on the lookout for warts and other symptoms; no treatment or evaluation is indicated in absence of clinical manifestations
• HPV immunization is recommended for sexually active persons <26 years old, including those with
clinical manifestations of HPV (e.g., genital warts, abnormal Pap smear)
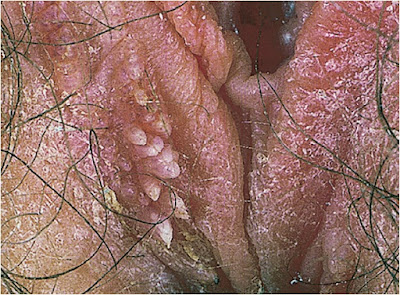
9–1. Genital warts of labia majora and perineum, with features of both
condylomata acuminata and keratotic warts.
CASE 1
Patient Profile Age 23, single graduate student
History Painless genital “bumps” for 1 month; monogamous in a relationship that began 4 months
earlier
Examination “Cauliflower-like” excrescences, some with horny surface, of labia majora and perineum
Diagnosis Genital warts (condylomata acuminata and keratotic warts)
Laboratory Routine screening for other STDs, including serological tests for syphilis and HIV, cervical NAATs for C. trachomatis and N. gonorrhoeae; Pap smear done 4 months earlier, not repeated
Treatment Cryotherapy with liquid nitrogen; repeat treatments scheduled weekly until resolved
Partner Management Patient was advised to refer partner if he noticed genital warts or other lesions, otherwise no need for examination
Comment The patient expressed concern about cancer risk and was counseled that warts and cervical
and other cancers are caused by separate HPV types. She was advised to continue routine Pap smears
as directed by her reproductive health provider, and to report persisting or recurrent external genital
lesions. She was further advised that most HPV infections eventually resolve (with or without treatment) and that late recurrence is possible but uncommon.
9–2. Multiple condylomatous and papular penile warts.
CASE 2
Patient Profile Age 26, single bookstore manager
History Painless penile growths for 6 weeks; 3 sex partners in past year
Examination Several 1- to 5-mm excrescences of penile shaft, some appearing “cauliflower-like,” others
smooth
Laboratory Screening tests for syphilis, gonorrhea, chlamydial infection, and HIV (all negative)
Diagnosis Genital warts (condylomata acuminata and papular warts)
Treatment Cryotherapy with liquid nitrogen, followed by self-treatment with podofilox gel 0.5% for
up to 4 weeks
Comment The patient was about to move to another city and requested continued self-applied therapy instead of returning for repeat cryotherapy. He was advised to inform partners of his diagnosis, who should seek examination if warts become apparent.
9–3. Anal warts (condylomata acuminata).
CASE 3
Patient Profile Age 43, HIV-infected male television cameraman
History Anal “bumps” and itching for 1 week; history of anal warts that resolved after treatment with
podophyllin 6 years earlier; monogamous with male partner for 2 years, denied receptive anal intercourse in past few months; HIV infection diagnosed 2 years earlier, with intermittent medical followup (not taking antiretroviral therapy); denied fever, weight loss, or other systemic symptoms
Examination Numerous anal and perianal excrescences with predominantly “cauliflower-like” morphology; anoscopy normal, without visible mucosal warts; general physical examination normal
Differential Diagnosis Anal warts; rule out anal cancer and syphilis (condylomata lata)
Laboratory Syphilis serology (negative); rectal cultures for N. gonorrhoeae and C. trachomatis negative
Diagnosis Anal condylomata acuminata; HIV infection
Treatment Cryotherapy with liquid nitrogen, repeated weekly
Comment The patient was referred to a proctologist for reassessment of intrarectal lesions and for consideration of biopsy to exclude anal dysplasia and cancer. He was advised that reappearance of warts might suggest progression of immunodeficiency and was referred to a local HIV/AIDS clinic to resume regular health care for HIV infection.
9–4. Condylomata acuminata of the vaginal introitus, with keratotic changes
at the terminus of the largest wart. Central venules can be seen in individual fronds.
9–5. Genital warts of scrotum. a. Condylomata acuminata and papular warts. b. Cryotherapy with liquid nitrogen.
9–6. Giant warts (Buschke-Löwenstein
tumor) of vulva. Malignant changes sometimes occur,
and such lesions should be
biopsied. Secondary infection and infarction
necrosis are common complications.
9–7. Neglected penile warts progressing to
giant condylomata.
giant condylomata.
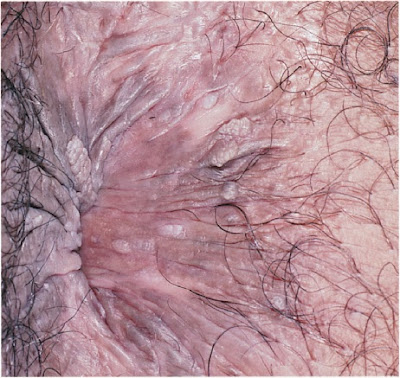
9–8. Papular warts of vaginal introitus and perineum.
9–9. Nodular pigmented lesions of vulvar
intraepithelial neoplasia (VIN) (white
arrow). The patient also has benign
condylomata acuminata (black arrow).
intraepithelial neoplasia (VIN) (white
arrow). The patient also has benign
condylomata acuminata (black arrow).
9–10. Cervix with subclinical flat wart. a. Normal-appearing ectocervix. b. Flat wart revealed by application of 3% acetic acid, viewed in green light. Note that flat wart does not coincide with the area of relative pallor observed before acetic acid application. Mucopurulent exudate is present in cervical os; C. trachomatis was isolated.
9–11. Large condylomata acuminata of rectal mucosa, viewed by anoscopy
9.12. Large condylomata acuminata of rectal mucosa, viewed by anoscopy
REFERENCES
H. Hunter Handsfield, MD, Color Atlas & Synopsis of Sexually Transmitted Diseases, Third Edition.





















COMMENTS